Covid-19 originated in mink and on the mink farms, and then spilled over to other animals and humans.
- Get link
- X
- Other Apps
The Hypothesis: Did COVID-19 originate on the mink farms?
The Hypothesis: Did COVID-19 originate on the mink farms? - Google Search
This article below is of the same opinion and it elaborates this hypothesis further.
A lot of work is needed to confirm or refute this hypothesis but it should remain the working assumption.
"Notably, there is limited evidence of animal-to-human transmission of SARS-CoV-2 except for mink."
A mutated strain of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been found in mink farms, such as this one in Naestved, Denmark, causing a mass cull in November 2020.
PHOTO: MADS CLAUS RASMUSSEN/RITZAU SCANPIX/AFP VIA GETTY IMAGES
Severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), and COVID-19 all broke out in recent decades and are caused by different strains of coronavirus (CoV). These viruses are considered to originate from bats and to have been transmitted to humans through intermediate hosts. SARS-CoV was identified in palm civets in wildlife markets and MERS-CoV in dromedary camels (1), but the direct source of the COVID-19 causative agent, SARS-CoV-2, is still undetermined. On page 172 of this issue, Oude Munnink et al. (2) report an in-depth investigation of SARS-CoV-2 infections in animals and humans working or living in 16 mink farms in the Netherlands. SARS-CoV-2 infections were detected in 66 out of 97 (68%) of the owners, workers, and their close contacts. Some people were infected with viral strains with an animal sequence signature, providing evidence of SARS-CoV-2 spillover back and forth between animals and humans within mink farms.
Besides mink, multiple species of wild or domestic animals may also carry SARS-CoV-2 or its related viruses. Experimental infections and binding-affinity assays between the SARS-CoV-2 spike (a surface protein that mediates cell entry) and its receptor, angiotensin-converting enzyme II (ACE2), demonstrate that SARS-CoV-2 has a wide host range (3). After the SARS-CoV-2 outbreak, several groups reported SARS-related CoVs in horseshoe bats in China and in pangolins smuggled from South Asian countries, but according to genome sequence comparison, none are directly the progenitor virus of SARS-CoV-2 (4). Domestic cats and dogs, as well as tigers in zoos, have also been found to be naturally infected by SARS-CoV-2 from humans, but there is no evidence that they can infect humans, and so they are unlikely to be the source hosts of SARS-CoV-2 (4, 5).
To date, SARS-CoV-2 infections in mink farms have been reported in eight countries (the Netherlands, Denmark, Spain, France, Sweden, Italy, the United States, and Greece), according to the World Organisation for Animal Health (6). In addition to animal-to-human transmission in farms, cold food supplier chains are raising substantial concern. In various cities in China, several small-scale COVID-19 outbreaks caused by virus-contaminated uncooked seafood or pork from overseas countries have been documented. It was found that viral genome signatures in these outbreaks were different from the viral strains present in China (7, 8). There is evidence that SARS-CoV-2 can survive up to 3 weeks in meat and on the surface of cold food packages without losing infectivity (7, 8). Thus, meat from SARS-CoV-2–infected animals or food packaging contaminated by SARS-CoV-2 could be a source of human infection (see the figure).
This raises concerns about public health and agriculture in the prevention and control of SARS-CoV-2. Most SARS-CoV-2–infected animals do not display an obvious clinical syndrome, and infections would be unrecognized without routine diagnosis. The massive mink culling of infected farms is an efficient way to prevent further transmission of the virus. However, it cannot be applied to all domestic animals (if other species are found to be SARS-CoV-2 hosts). Thus, out of caution, extensive and strict quarantine measures should be implemented in all domestic farms with high-density animal populations. Because the virus is able to jump between some animals (such as mink) and humans, similar strategies should be applied to people in key occupations involving animal-human interfaces, such as animal farmers, zookeepers, or people who work in slaughterhouses. Notably, there is limited evidence of animal-to-human transmission of SARS-CoV-2 except for mink. Research on whether other domestic animals carry SARS-CoV-2, whether they can transmit it to humans, and factors related to spillover should be conducted.
The RNA genome of SARS-CoV-2 seems relatively stable during transmission within human populations, although accumulated mutations have been detected. It is generally accepted that coronaviruses tend to exhibit rapid evolution when jumping to a different species. To keep the replication error rate low, coronaviruses encode several RNA-processing and proofreading enzymes that are thought to increase the fidelity of viral replication. However, viruses tend to have reduced fidelity in favor of adaptation to a new host species (9), although the mechanisms underlying this phenomenon are unclear. The coronaviral spike protein is prone to have more mutations because it is the first virus-host interaction protein and thus faces the strongest selection pressure. This molecular evolution can be observed in SARS-CoV genomes, which were under more adaptive pressure in the early stage of the epidemic (palm civet to human) than in later stages (human to human) (10).
Mutations that occur in SARS-CoV-2 in animals may increase its pathogenesis or transmissibility in humans. Five clusters of SARS-CoV-2 strains were found in mink, each characterized by a specific mink-related variant. In Denmark, the cluster 5 strain of mink SARS-CoV-2 was less immunogenic to COVID-19 patient serum than was human SARS-CoV-2 because of mutations of the spike proteins in the mink strains (11). This cluster 5 strain has infected at least 12 people, and the clinical presentation, severity, and transmission among those infected are similar to those of other circulating human SARS-CoV-2 strains (12). Currently, there is no evidence that any mutation from mink strains of SARS-CoV-2 escapes neutralization by antibodies designed to target the prevalent human strains. However, considering the possible risk of spillover of SARS-CoV-2 between humans and some animals, it is imperative to closely monitor mutations in the viral genome from infected animals and humans, particularly the genome regions affecting diagnostic tests, antiviral drugs, and vaccine development.
It is anticipated that vaccines will allow control of COVID-19. Vaccines have been developed against the current prevalent viral strains and could face challenges if there is continued spillover from animals. The viral genome mutations likely produced during interspecies transmission between animals and humans raise concerns about whether the current vaccines can protect against emerging strains in the future. The extensive sequencing of viral genomes from animals and humans and worldwide data sharing will be central to efforts to monitor the key mutations that could affect vaccine efficacy. Laboratory-based studies should test whether the observed mutations affect key features of the virus, including pathogenesis, immunogenicity, and cross-neutralization. Moreover, preparedness of vaccines based on newly detected variants should be considered in advance. In the long term, vaccination of animals should also be considered to avoid economic losses in agriculture.
There has been debate about whether bats or pangolins, which carry coronaviruses with genomes that are ∼90 to 96% similar to human SARS-CoV-2, were the animal source of the first human outbreak (4). Evolutionary analyses of viral genomes from bats and pangolins indicate that further adaptions, either in animal hosts or in humans, occurred before the virus caused the COVID-19 pandemic (13). Therefore, an animal species that has a high population density to allow natural selection and a competent ACE2 protein for SARS-CoV-2—mink, for example—would be a possible host of the direct progenitor of SARS-CoV-2.
Another debate concerns the source of SARS-CoV-2 that caused the COVID-19 outbreak at the end of 2019. The current data question the animal origin of SARS-CoV-2 in the seafood market where the early cases were identified in Wuhan, China. Given the finding of SARS-CoV-2 on the surface of imported food packages, contact with contaminated uncooked food could be an important source of SARS-CoV-2 transmission (8). Recently, SARS-CoV-2 antibodies were found in human serum samples taken outside of China before the COVID-19 outbreak was detected (14, 15), which suggests that SARS-CoV-2 existed for some time before the first cases were described in Wuhan. Retrospective investigations of preoutbreak samples from mink or other susceptible animals, as well as humans, should be conducted to identify the hosts of the direct progenitor virus and to determine when the virus spilled over into humans.
References and Notes
- ↵
- J. Cui,
- F. Li,
- Z. L. Shi
- ↵
- B. B. Oude Munnink et al
- ↵
- L. Wu et al
- ↵
- B. Hu,
- H. Guo,
- P. Zhou,
- Z. L. Shi
- ↵
- D. McAloose et al
- ↵
- ↵
- P. Liu et al
- ↵
- J. Han,
- X. Zhang,
- S. He,
- P. Jia
- ↵
- R. L. Graham,
- R. S. Baric
- ↵Chinese SARS Molecular Epidemiology Consortium, Science 303, 1666 (2004).
- ↵
- ↵
- ↵
- K. G. Andersen,
- A. Rambaut,
- W. I. Lipkin,
- E. C. Holmes,
- R. F. Garry
- ↵
- G. Apolone et al
- ↵
- S. V. Basavaraju et al
Acknowledgments: Supported by China National Science Foundation for Excellent Scholars award 81822028 (P.Z.) and Strategic Priority Research Program of the Chinese Academy of Sciences awards XDB29010101 (Z.-L.S.) and XDB29010204 (P.Z.).
Shi Zhengli. Photo: Wuhan Institute of Virology (WIV)
While the World Health Organization's expert team is in Wuhan to investigate how the coronavirus jumped to humans from animals, China's "Bat Woman" Shi Zhengli said in an article that mink could be a possible host of the origin of the novel coronavirus, and called on the world to investigate samples from more susceptible animals to determine when the virus moved to humans.
Chinese virologists said Shi's article proves that the mystery of the virus origin needs investigations in many countries and research on more animal species, and WHO's investigation in Wuhan, although unlikely to find the answer of the origin, will lay a good foundation for further investigations in more countries.
However, Chinese experts also stressed that Shi's article did not fix the direct host of the virus on mink, and scientists in different fields need to deepen their genome sequence research on mink to verify the possibility.
Shi, a Chinese virologist from the Wuhan Institute of Virology dubbed as China's "Bat Woman" due to her years of research and achievements in research with bats and viruses, and Zhou Peng, a scientist also from the institute, jointly published an article, titled "SARS-CoV-2 spillover events," on Science Magazine recently, which said that after the COVID-19 outbreak in Wuhan, several groups reported SARS-related coronavirus in horseshoe bats in China and in pangolins smuggled from South Asian countries. But according to genome sequence comparison, none are directly the source of SARS-CoV-2, the agent of COVID-19.
In the meantime, COVID-19 infections in mink farms have been reported in eight countries, including the Netherlands, France, Italy and the US, according to the WHO.
There is limited evidence of animal-to-human transmission of SARS-CoV-2 except for mink, the study said.
Evolutionary analyses of viral genomes from bats and pangolins indicate that further adaption, either in animal hosts or in humans, occurred before the virus caused the COVID-19 pandemic, the study said. Therefore, an animal species that has a high population density, for example, mink, would be a possible host of the origin of SARS-CoV-2.
The current evidence also questioned the animal origin of the virus in the seafood market in Wuhan, as we have found the virus on the surface of imported food packages, and the coronavirus antibodies were found in human serum samples taken outside China before the COVID-19 outbreak was detected in Wuhan, which suggested that the virus had existed for some time before Wuhan discovered its first case, the study said.
Authors of the study called on retrospective investigations of pre-outbreak samples from mink and other susceptible animals as well as humans to identify the direct origin of the virus, and to determine when the virus moved to humans.
Shi's study was published amid the WHO expert team's investigation trip to Wuhan, which Chinese health experts believe would help worldwide experts explore more possibilities on the origin of the virus.

WHO Photo:VCG
WHO's mission
According to a Terms of References for the China part on the global study of virus origin sent to the Global Times by the WHO ahead of the visit, the international team will conduct studies to better understand how the virus might have started circulating in Wuhan, and these studies include in-depth reviews of hospital records for cases compatible with COVID-19 before December 2019, and a mapping of activities and items traded at the Huanan seafood market and potentially other relevant markets in late November and December 2019, including types of animals and stalls present at the Huanan market, according to the WHO.
Yang Zhanqiu, deputy director of the pathogen biology department at Wuhan University, told the Global Times on Friday that the WHO experts could work with Chinese counterparts to learn how and why the virus circulated in Wuhan, and their visit to Wuhan, including their methods and other experience, will set a fine example for more investigations in more countries.
Jin Dongyan, a biomedical professor at the University of Hong Kong, said that the WHO experts consisting of international scientists in various fields could provide China different experiences and thoughts on related studies.
But the world cannot pin their hopes of finding how the virus jumped from animals to humans and which species it came from solely on the ongoing WHO experts' visit to China, which most likely will find no answer at all.
Too many scientific questions remain unanswered, such as how the virus triggered humans to develop the symptoms, and the infection chain between animals and humans. These answers cannot be found during Wuhan's visit, Yang said.
The COVID-19 outbreak was first reported in Wuhan, but where an epidemic is first detected does not reflect where it started, according to the WHO.
Countries including the US, Italy and France have reported coronavirus evidence in environmental and human specimens before or around the time the virus was officially identified in China in December 2019.
Fabian Leendertz, who is part of the WHO expert team and biologist at Germany's Robert Koch Institute, told the Global Times previously that the team would start where the first solid evidence was generated, and this is in Wuhan.
"From there we do the scientific work and follow the tracks wherever they take us... could be within China, could be outside China. We have to be open to all directions, this is what science is about and it is data based," Leendertz said.
After overturning the possibility of bats, Shi's article provided a new possibility, although more thorough research is needed, and helped broaden the range of potential animal hosts, experts said.
Yang said it's possible we missed some animals in hunting the virus source because no scientists have dug into them.
"They may not just live in China but in Africa, the Americas or Europe. Is it possible that some animals in places such as the US where natural ecological environment is well-protected have some relation to the novel coronavirus as well?" Yang said.
So far, mutations of the virus have been found in the US, not Wuhan, and the WHO experts need to investigate in the US, Yang said.
The visit of WHO experts should also highlight sharing the Chinese experience on controlling the pandemic in a timely manner. This is a more realistic mission to complete, Yang said.
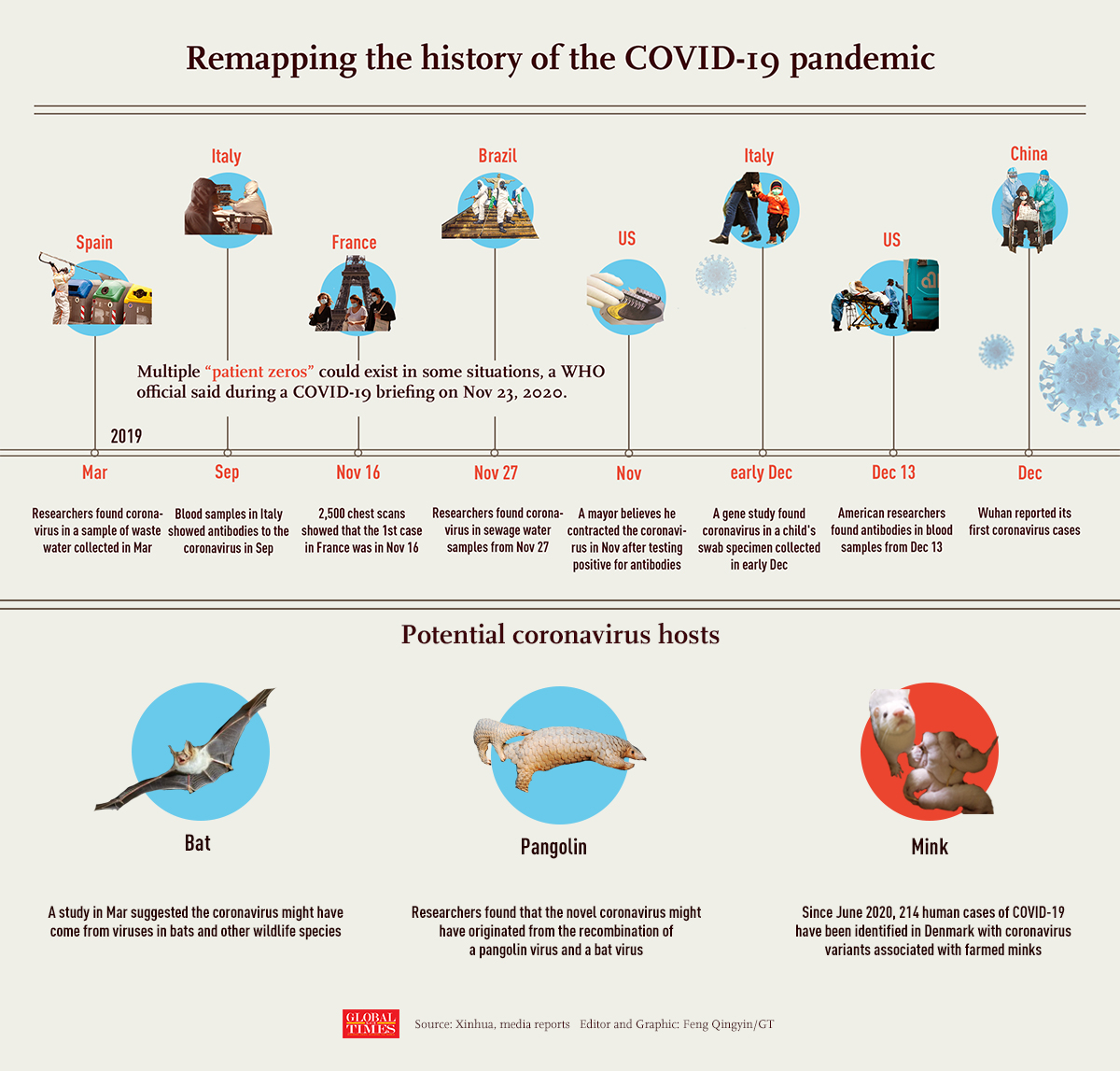
Remapping the history of the COVID-19 pandemic
The coronavirus that conquered the world came from a thumb-sized bat tucked inside a remote Chinese cave. Of this much, scientists are convinced.
Exactly how and when it fled the bat to begin its devastating flight across the globe remain open questions.
In just one year, SARS-CoV-2, the virus that causes COVID-19, has infected 100 million people and killed 2 million, 400,000 of them in the U.S. Answers could stop such a calamity from happening again.
Researchers in China, under government scrutiny, have been investigating since January. This week, a World Health Organization delegation of scientists from 10 different nations finally was allowed in the country to explore the virus' origins.
"This is important not just for COVID-19, but for the future of global health security and to manage emerging disease threats with pandemic potential," Tedros Ghebreyesus, WHO's director-general, said just after the team left for China.
It's not clear how much evidence will remain a year later, and what the team will be able to learn. The Wuhan fish market, seen as a likely breeding ground for the virus, has been scrubbed and shuttered.
But the effort is worth it, infectious disease experts say. Understanding the journey of SARS-CoV-2 may provide insights into how the relationship between humans and animals led to the pandemic, as well as other disease outbreaks including Ebola, Zika and many strains of flu.
"These are emerging diseases that breach the barrier between animals and humans and cause devastation in human populations," the WHO's Mike Ryan said at a Monday news conference. "It is an absolute requirement that we understand that interface and what is driving that dynamic and what specific issues resulted in diseases breaching that barrier."
The international team is not looking to assign blame, said Ryan, executive director of WHO's Health Emergencies Programme. If it were, there would be plenty to go around.
"We can blame climate change. We can blame policy decisions made 30 years ago regarding everything from urbanization to the way we exploit the forest," he said. "You can find people to blame in every level of what we're doing on this planet."
The chain of events that led to the worst global pandemic in a century started with a tiny, insect-eating mammal with the mundane name, Intermediate Horseshoe bat.
The species is part of a family of bats that act as natural reservoirs for coronaviruses, notorious for how easily they mutate and how well they can be transmitted from species to species. The bats aren't bothered by the viruses. The animals they pass them onto aren't always so lucky.
Humans are one of those animals.
This happens all the time – a virus harmlessly infects one creature then finds its way to another, mutates and becomes something new. The newly mutated virus can be insignificant but annoying (think common colds, some of which are caused by coronaviruses) or devastating and deadly (think smallpox.)
SARS-CoV-2 is a little of both.
As many as 40% of those who test positive for COVID-19 have no symptoms at all but 2% of people who get sick die. It’s especially deadly in the elderly. COVID-19 has killed 1 of every 66 Americans older than 85. Among those infected, some percentage — we don't yet know how many — cope with crippling long-term symptoms that plague them for months. Future health impacts remain unknown.
The group of related coronaviruses giving rise to SARS-CoV-2 has existed for decades in bats and likely originated more than 40 years ago, said Dr. Charles Chiu, a professor and expert in viral genomics at the University of California, San Francisco.
SARS-CoV-2 shares 96% of its genetic material with a sample of coronavirus taken in 2013 in Intermediate Horseshoe bats from Yunnan province in China, which suggests the Yunnan virus is its ancestor. How the virus traveled the 1,200 miles from Yunnan to Wuhan remains unknown.
Because the 2013 sample is the only one available, scientists had to undertake genetic analysis to estimate when the bat strain and the strain now circulating among humans diverged. They put the split sometime in the 1960s or 1970s, said Maciej Boni, a professor of biology at Pennsylvania State University's Center for Infectious Disease Dynamics, who spent almost a decade working in Asia.
"There's really not a clear tree where we have forensic evidence to point to exactly where it came from," said John Connor, a virologist at Boston University who studies emerging infectious diseases. "It looks like it's a bat-derived virus, and there's a big question mark after that."
Scientists simply don't do enough surveillance of bats and coronavirus to tell.
"We just don't know because we don't have any data — we weren't looking," said Boni. "Over the last 20 years we haven't been doing enough sampling."
Boni is among those who think the virus most likely came directly from bats, possibly infecting miners who work in bat-infested caves or people exposed to bat feces. Others say it more likely spent some time infecting another animal species before leaping to humans.
The original SARS virus, identified in China in 2003, is believed to have passed through civets – a type of nocturnal mammal native to Asia and Africa – though other animals may have been involved.
SARS underwent only a few genetic changes between bats and people, which made its animal roots easier to trace, while SARS-CoV-2 has changed a lot more, Connor said.
With SARS-CoV-2, a suspect is the frequently trafficked scaly anteater, also known as a pangolin. Other possibilities include civets or ferrets or even cats.
“SARS-CoV-2 may originate from live animal markets, but it may also have emerged from any setting in which people come into contact with animals, including farms, pets, or zoos,” Chiu said.
Whatever its path, sometime before November 2019 it became a virus that could easily – far too easily – infect humans.
Despite a persistent conspiracy theory that SARS-CoV-2 was developed in a lab, perhaps an infectious disease lab in Wuhan, there’s no evidence to support the claim and plenty to counter it.
In March, a group of researchers found the virus most closely resembled existing bat viruses and was not man-made.
"Our analyses clearly show that SARS-CoV-2 is not a laboratory construct or a purposefully manipulated virus," they wrote in the prestigious journal Nature.
No new details have emerged since to change the author minds, said Dr. W. Ian Lipkin, one of the co-authors and a professor at the Columbia University Mailman School of Public Health.
"Can we exclude the possibility that there was a virus that was present in this lab that somehow got out into either animals or people? No, we can't do that," he said. "The only thing we can say is that there's no evidence that suggests it was deliberately engineered through some sort of gain-of-function experiments."
Connor said he's also dubious the virus originated in a lab rather than in nature.
"What laboratory people are really good at doing is making viruses weaker," said Connor, who is also an investigator at Boston University's National Emerging Infectious Disease Laboratories.
Viruses, especially RNA viruses like coronaviruses, make tiny mistakes as they reproduce. One person's nose might contain 10 to a 100,000 copies of the virus, and with so many replications and so many mistakes, it's plausible chance mutations led to SARS-CoV-2, he said.
"I don't think we need to look for man-made. I think we see the viruses that we know assaulting us all the time," Connor said. "We look back to Zika. That wasn't man-made. Neither was Ebola. Flu keeps coming after us."
It’s possible to bioengineer a virus, but it’s extremely hard. Anyone doing so would have used a pre-existing virus as the template. The virus that’s now killing millions has novel mutations, many of them, said Chiu.
“We barely know how to manipulate even a few base pairs in a single viral gene," he said. "The difference between Chinese bat coronaviruses and SARS-CoV-2 is more than 3,000 base pairs."
In some ways, it doesn't matter where the virus came from, said Stephen Morse, a professor of epidemiology at Columbia University's Mailman School of Public Health. What matters is how we deal with the current situation, which is at a crisis state in the United States.
"When the house is burning down is not the time to start looking for where the matches were," he said.
If SARS-Cov-2 had been a type of bird flu instead of a coronavirus, the world would have alerted within days of the first infections. A global surveillance system was established in the 1990s and has been expanded and strengthened, Boni said.
"If a single poultry farmer in Southeast Asia comes down with severe respiratory symptoms, samples are taken and sequenced. That week you know which avian influenza virus it is," he said. "Farms in neighboring regions are immediately quarantined and the birds may be depopulated. It takes days."
Setting up something similar for bats and coronaviruses would cost several billion a year globally, said Boni. "It's not expensive for the benefit we'd get."
To track SARS-COV-2 as it transferred among species requires analyzing blood collected from the animals, as well as samples from their airways.
Distinguishing between closely related viruses isn't always so easy.
"We have a special test that can do this if we could get samples out of China," said Lipkin. He's been trying for months to do so, and when he attempted to send his own sampling tools into the country the U.S wouldn't allow it.
"We now have obstruction on both sides," said Lipkin, who's been working to get into China himself since early in the outbreak. "I don't know when that's going to let up. I'm hoping the Biden administration will feel differently."
Lipkin's March paper explored key features of the new virus but nothing more has been learned since about SARS-CoV-2's earliest days, he said.
"We still haven't had a full post-mortem on what went wrong in China," said Lipkin, who caught COVID-19 in March in New York and was recently vaccinated.
The U.S. has a very good system of reporting outbreaks, and rapidly publishes information in the CDC's journal, Morbidity and Mortality Weekly. The Chinese are not as transparent at reporting their public health information.
Increased transparency is one of several changes Lipkin recommends to avoid a repeat of the 2020 disaster.
Wild animal markets and consumption of wildlife continue to pose dangers, he said.
And the world needs to have the ability to respond faster to novel viruses like SARS-CoV-2. Global surveillance would help, as would drugs that can treat a wide spectrum of viruses – maybe one that can address all coronaviruses and another to tackle influenzas.
"These drugs might not be ideal but we should think of them as a finger in the dike," Lipkin said, so outbreaks won't get out of hand, the way this one did.
Connor, at Boston University, agrees that effective and transparent public health systems around the world are essential for detecting and preventing outbreaks like COVID-19.
While Wuhan may have had a good health care system, that was not the case in West Africa, where a 2014-2016 epidemic of Ebola infected more than 28,000, killed over 11,000 and terrified the world.
"It would be nice for all people to have good health care, not just because it would be nice for them ... but for everybody else," Connor said. "It would be nice to be able to identify: Oh, all of a sudden, five people in one area got sick with something we didn't know what it was."
Connor said it's pointless to try to predict all the ways in which a virus now infecting animals could make the leap to humans. A much better approach, he said, is to focus on the viruses that do emerge.
"What matters is how good we are at responding quickly," he said.
The race is now between the speed of mutations and the speed of vaccination, said Chiu.
Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, says it may take up to 85% of Americans being vaccinated to protect the population. Reaching those numbers will be challenging considering pervasive vaccine hesitancy and a slow, complicated roll out.
In the meantime, public health measures to stop the spread – masking, social distancing and handwashing – are essential, experts repeat.
“We have to reduce the number of infections before the virus has a chance to mutate in such a way that it can evade drugs and vaccines," said Chiu. "That’s what keeps me up at night.”
Contact Elizabeth Weise at <a href="mailto:eweise@usatoday.com">eweise@usatoday.com</a> and Karen Weintraub at <a href="mailto:kweintraub@usatoday.com">kweintraub@usatoday.com</a>.
Health and patient safety coverage at USA TODAY is made possible in part by a grant from the Masimo Foundation for Ethics, Innovation and Competition in Healthcare. The Masimo Foundation does not provide editorial input.
- Get link
- X
- Other Apps



Comments
Post a Comment